Abstract
Introduction: Flotetuzumab (FLZ) is a bispecific CD123 x CD3 DART® molecule that is being investigated for the treatment of relapsed and refractory acute myeloid leukemia (AML). FLZ induces artificial immunological synapses between CD3 on cytotoxic T-cells and CD123, which is expressed on AML blasts along with off-target hematopoietic and non-hematopoietic tissues. T-cell activation and expansion requires cytokine release from both innate and adaptive immune cells, which when excessive, can result in cytokine release syndrome (CRS), a potentially life-threatening toxicity that manifests with fever, hypotension, organ dysfunction, respiratory failure, and/or coagulopathy. FLZ-mediated anti-leukemia efficacy is often limited by CRS. Therefore, developing pre-clinical models by which to better understand the mechanisms of CRS induction, including collaborating cell types that produce IL-6 and other inflammatory cytokines, is essential to understanding the biology of CRS and developing mechanistically plausible preventative and treatment strategies. MacroGenics has developed a murine genetic knock-in model that expresses a chimeric murine CD3 epsilon (CD3e) chain bearing the human CD3e epitope recognized by FLZ (hCD3e). These mice are immune competent, maintain normal signaling through the T-cell receptor and expand and secrete cytokines upon binding to FLZ and human CD123. To validate this model, we explore whether T-cells harvested from the spleens of immune competent C57Bl/6 hCD3e mice will bind FLZ and kill human CD123-expressing target cells in vitro.
Methods: A murine AML cell line (AML1-GFP FLT3-ITD, DNMT3AR878H) was transduced with human CD123 (hCD123) using a lentiviral vector construct and single cell clones expressing high levels of hCD123 were expanded (AMLGFP-hCD123). Spleens from hCD3eHOM C57Bl/6 mice were harvested for isolation of T-lymphocytes via negative immunomagnetic selection on an AutoMACS machine. In vitro killing assays were set up using purified C57Bl/6 hCD3eHOM T-cells and two clones of AMLGFP-hCD123 cells. The effector to target ratio was 1:1, with 2.5x105 cells per well in a 24-well plate. Flow cytometry/FACS was then performed at 24 hours to assess killing of AMLGFP-hCD123 clones 4 and 14 and T-cell subset number and phenotype. Antibodies to the following antigens were used for FACS: mCD4, hCD3 (epitope SP38), mCD25, mCD8, mCD44, mCD62L, hCD123, mCD3, mCD127, mCD69, and mCD45, in addition to endogenous GFP.
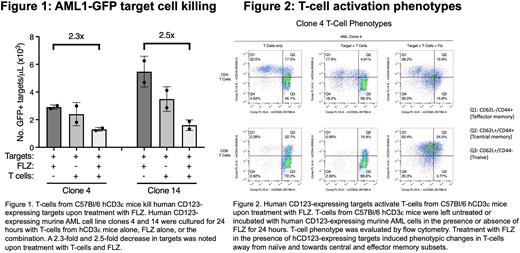
Results: At 24 hours, target cell killing of clones 4 and 14 was noted (Figure 1). Compared to the controls treated with FLZ or T-cells alone, targets treated with T-cells + FLZ showed a 2.3- and 2.5-fold decrease in the number of AMLGFP-hCD123 targets in clones 4 and 14, respectively, at 24 hours (Figure 1). In terms of T-cell phenotypic changes, the percentage and MFI expression of activation markers, namely CD25 and CD69, increased for CD4+ and CD8+ subsets in the treatment group compared to controls (data not shown). T-cell subsets were characterized as naïve (CD62L+/CD44-), central memory (CD62L+/CD44+), and effector memory (CD62L-/CD44+), as shown in Figure 2. For CD4+ and CD8+ subsets co-cultured with targets, the addition of FLZ induced phenotypic changes away from naïve and towards central and effector memory subsets (Figure 2). In the two control groups, naïve T-cells predominated at the 24-hour time point (Figure 2).
Conclusions: The in vitro killing assay shows that T-cells from the spleens of immune competent C57Bl/6 hCD123eHOM mice recognize FLZ and hCD123 on transduced murine leukemia cells. Co-culture with T-cells + target cells + FLZ showed killing at 24 hours compared to controls. T-cell activation and proliferation were noted based on cell surface markers. The shift in phenotype from naïve to central and effector memory subsets suggests T-cell expansion upon leukemia antigen recognition and portends killing of target cells. Pending in vivo studies will evaluate if FLZ can activate endogenous T cells, induce CRS, and kill AMLGFP-hCD123 targets in the huCD3eHOM mice. If successful, we will use the huCD3eHOM model to test the efficacy of various therapies to mitigate CRS and preserve FLZ-mediated T-cell cytotoxicity against AMLGFP-hCD123 targets in huCD3eHOM mice.
Disclosures
Alderson:Macrogenics Inc.: Current Employment. Bonvini:MacroGenics, Inc: Current Employment, Other: company stocks as part of the compensation package. DiPersio:hC Bioscience, Inc.: Membership on an entity's Board of Directors or advisory committees; Amphivena Therapeutics: Research Funding; NeoImmune Tech: Research Funding; Macrogenics: Research Funding; BioLineRx, Ltd.: Research Funding; CAR-T cell Product with Washington University and WUGEN: Patents & Royalties; VLA-4 Inhibitor with Washington University and Magenta Therapeutics: Patents & Royalties; RiverVest Venture Partners: Consultancy, Membership on an entity's Board of Directors or advisory committees; Incyte: Consultancy, Research Funding; WUGEN: Current equity holder in private company, Research Funding; Magenta Therapeutics: Current equity holder in private company, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal